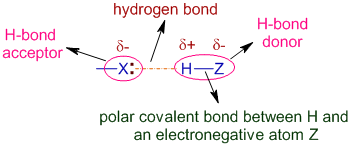
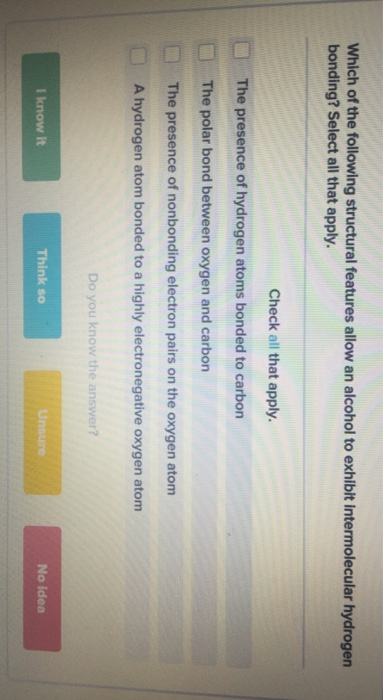
QUESTION: How many atoms in the acetic acid molecule (CH3COOH) are capable of hydrogen bonding interaction with water molecules? A. 1, B. 2, C. 3, D. - ppt download
Water Molecules Still Shrouded in Mystery - Unlocking the secrets of the mysterious structure and patterns of motion of water molecules — SPring-8 Web Site

Chemistry 11Chapter 4 Chemical Bonding & Molecular Structure Hydrogen Bond -Inter & Intra Molecular - YouTube

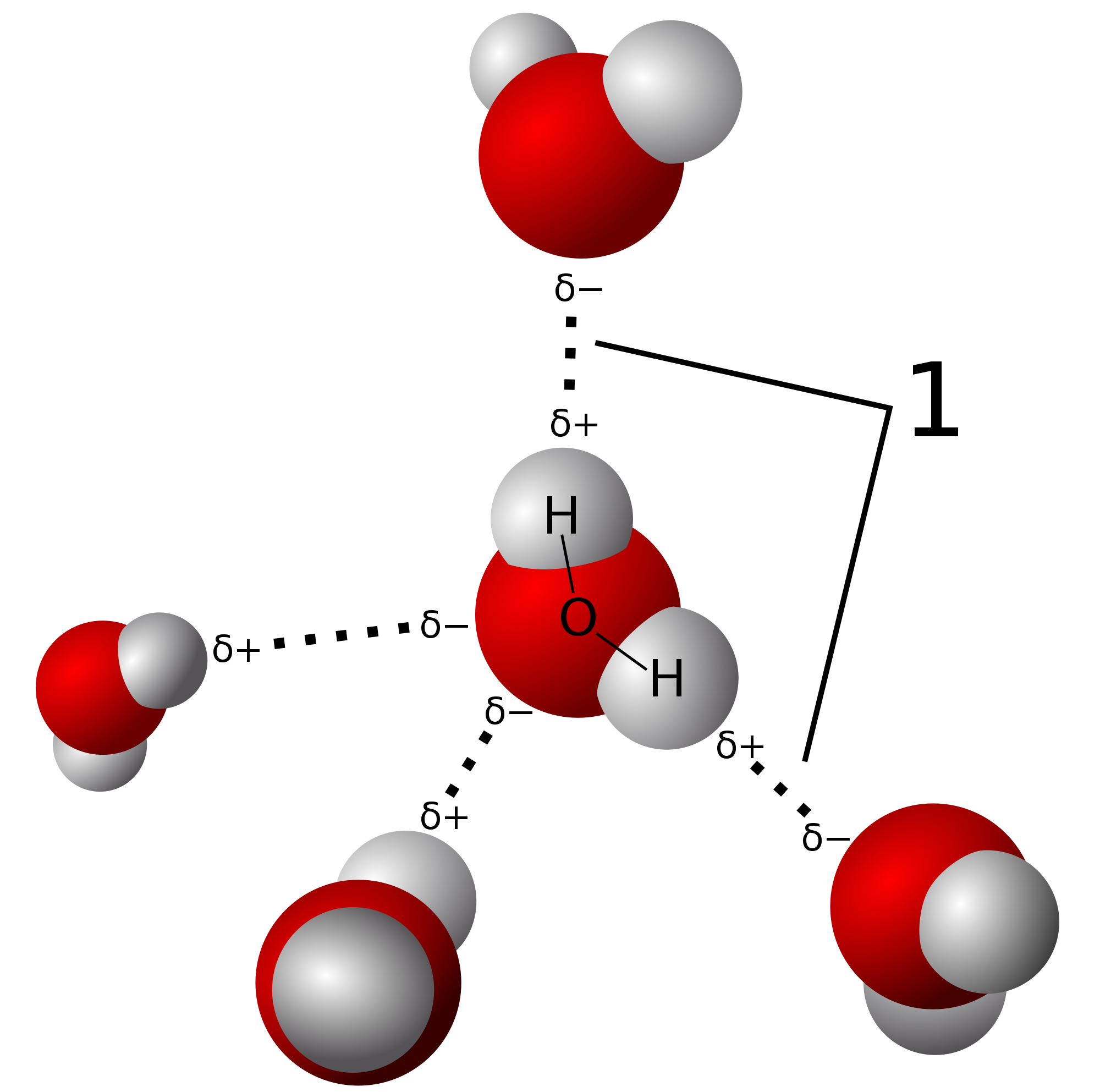
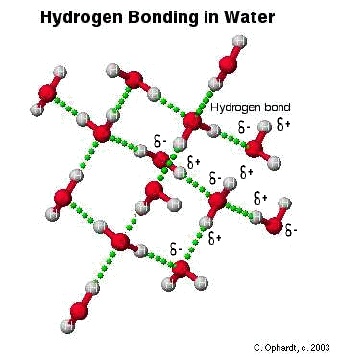
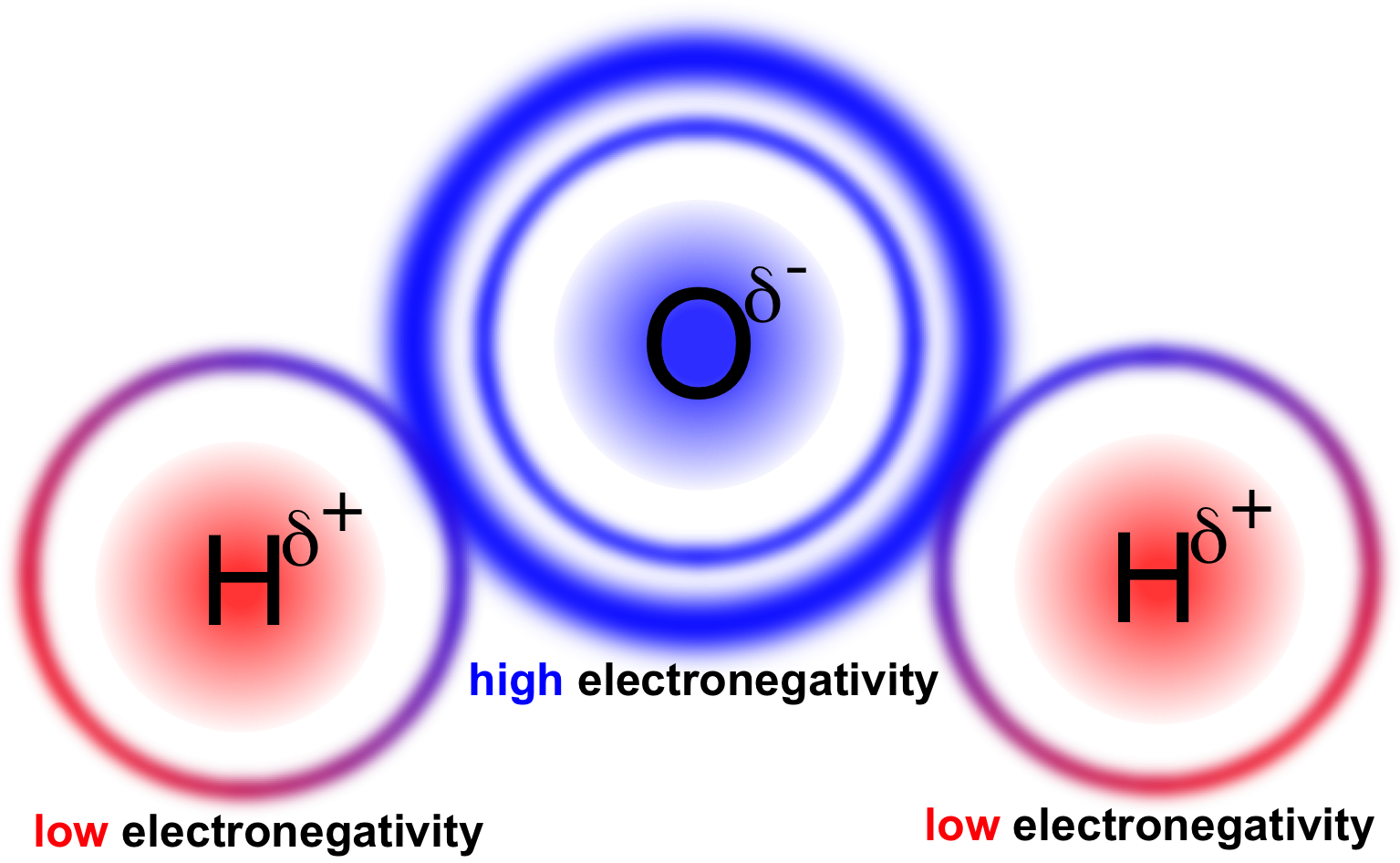
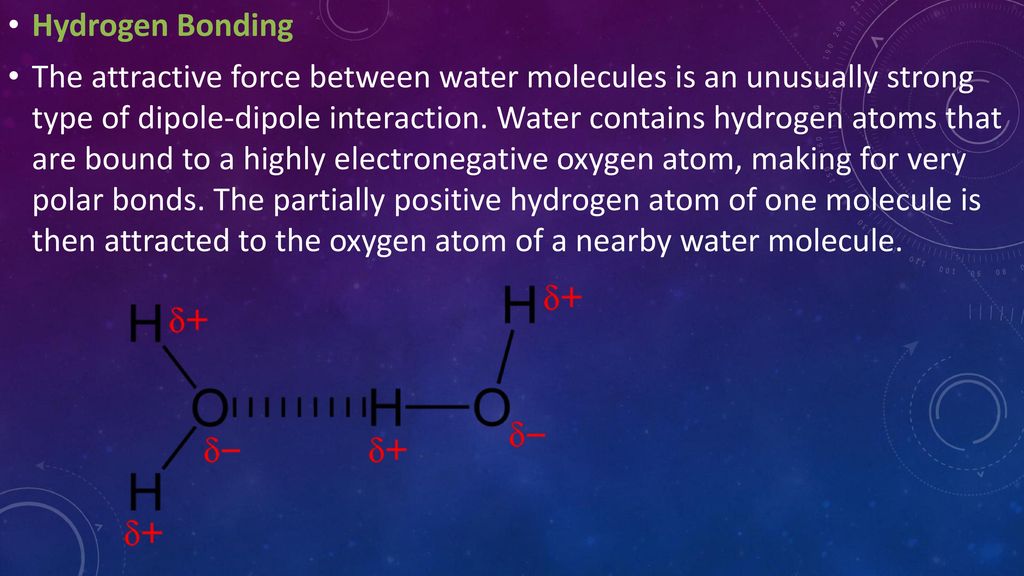
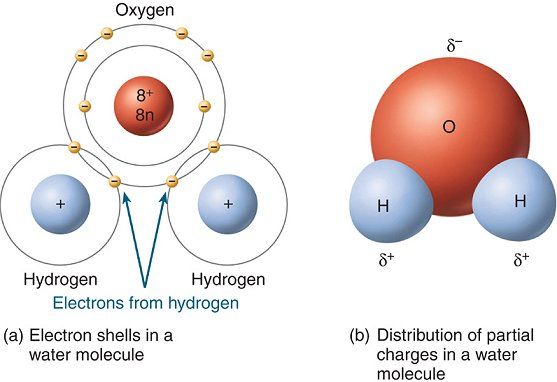
In a water molecule two hydrogen atoms form single polar covalent bonds with an oxygen atom. –Because oxygen is more electronegative, the region around. - ppt download










/PeriodicTableElectronegativity-56a12a045f9b58b7d0bca77c.jpg)